Description
Buy 1-Boc-4-AP Cas 125541-22-2
Buy 1-Boc-4-AP Cas 125541-22-2. 1-Boc-4-AP (tert-butyl 4-(phenylamino)piperidine-1-carboxylate) is a compound used as an intermediate in the manufacture of fentanyl, as well as various related derivatives such as butyrylfentanyl, furanylfentanyl, benzylfentanyl and homofentanyl, among others. It is an N-protected derivative of 4-anilinopiperidine which can be readily converted to fentanyl or related analogues in several straightforward synthetic steps. It was classified as a DEA List 1 Chemical in 2022, and is also controlled in various other jurisdictions. Its possession, sale and importation are consequently heavily regulated throughout much of the world. 1-Boc-4-AP has also been identified as an impurity in other designer drug products, though it is unclear if it has any pharmacological activity in its own right. Buy 1-Boc-4-AP Cas 125541-22-2
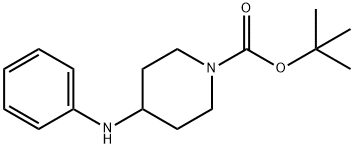
1-N-Boc-4-(Phenylamino)piperidine
- CAS No.
- 125541-22-2
- Chemical Name:
- 1-N-Boc-4-(Phenylamino)piperidine
- Synonyms
- tert-butyl 4-anilinopiperidine-1-carboxylate;1-Boc-4-(phenylaMino)piperidine;tert-butyl 4-(phenylaMino)piperidine-1-carboxylate;1-N-Boc-4-(Phenylamino);Aniline piperidine;(Phenylamino)piperidine;4-(Phenylamino)piperidine, N1-BOC protected;2-Methyl-2-propanyl 4-anilino-1-piperidinecarboxylate;4-(phenylamino)-;1-N-Boc-4-(Phenylami
- CBNumber:
- CB2262675
- Molecular Formula:
- C16H24N2O2
- Molecular Weight:
- 276.37
- MDL Number:
- MFCD02102489
- MOL File:
- 125541-22-2.mol
| Melting point | 136-137 |
|---|---|
| Boiling point | 400.6±38.0 °C(Predicted) |
| Density | 1.107±0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | DMF: 15mg/mL; DMSO: 25mg/mL; Ethanol: 25mg/mL; Ethanol:PBS (pH 7.2) (1:1): 0.5mg/mL |
| form | A crystalline solid |
| pka | 5.00±0.20(Predicted) |
| InChI | InChI=1S/C16H24N2O2/c1-16(2,3)20-15(19)18-11-9-14(10-12-18)17-13-7-5-4-6-8-13/h4-8,14,17H,9-12H2,1-3H3 |
| InChIKey | HTIWISWAPVQGMI-UHFFFAOYSA-N |
| SMILES | N1(C(OC(C)(C)C)=O)CCC(NC2=CC=CC=C2)CC1 |
| FDA UNII | 5PB6P54SRS |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P305+P351+P338 | |||||||||
| Hazard Codes | Xi,Xn | |||||||||
| Risk Statements | 22 | |||||||||
| Hazard Note | Harmful/Irritant | |||||||||
| HazardClass | IRRITANT | |||||||||
| HS Code | 2933399990 | |||||||||
| NFPA 704 |
|
Supply 99% 1-N-Boc-4-(Phenylamino) piperidine powder buy 125541-22-2
1-N-Boc-4-(Phenylamino) piperidine Basic info
Product name:1-N-Boc-4-(Phenylamino) piperidine
Alias:tert-Butyl 4-anilinopiperidine-1-carboxylate;4-Anilino-1-Boc-piperidine
CAS:125541-22-2
MF:C16H24N2O2
MW:276.37
Assay:99%min
Appearance:white powder
Packing:25KG/ cardboard drum or upon customers’ request.
1-N-Boc-4-(Phenylamino) piperidine uses:Pharmaceutical Intermediates
1-N-Boc-4-(Phenylamino) piperidine description:
1-N-Boc-4-(Phenylamino) piperidine function:
1-N-Boc-4-(Phenylamino) piperidine application:
used as a pharmaceutical intermediates.Intermediate in the preparation of Fentanyl derivatives.
1-N-Boc-4-(Phenylamino)piperidine Chemical Properties,Uses,Production
Description
4-Anilino-1-Boc-piperidine is an analytical reference standard that is structurally similar to known opioids. 4-Anilino-1-Boc-piperidine is a precursor in the synthesis of 4-anilinopiperidine . This product is intended for research and forensic applications.
Chemical Properties
Pale Brown Solid
Uses
Intermediate in the preparation of Fentanyl derivatives.
Application
1-N-Boc-4-(Phenylamino)piperidine is an amino acid derivative widely employed as a reagent in organic synthesis. This compound exhibits remarkable versatility, finding applications in various fields such as peptide and protein synthesis, organic synthesis, and drug development. It possesses strong basic properties, allowing for diverse reactions with acids that lead to the formation of a wide array of products. The extensive use of this compound in organic synthesis is well-documented, particularly in the synthesis of peptides and proteins. By facilitating the coupling of amino acids, it enables the creation of peptides that can be utilized in diverse applications, including the development of pharmaceutical drugs.








Reviews
There are no reviews yet.